The batteries in most of your high tech devices will be based upon Lithium-ion technology. Lithium is a rather rare metallic element mostly mined from a few areas in South America. If this source starts to run low then there could be severe implications to the technology, automotive industries, and others.
Simply moving down the periodic table to the next 'alkali metal' element points towards Sodium being a possible substitute for Lithium, and it does indeed share many chemical properties. If you remember high school chemistry it is even more violently reactive - would this be a positive (more energy stored?) or a negative (more dangerous?). Unfortunately it isn't as simple as weighing up and addressing these variables, due to interactions with other components in a battery.

Purdue researcher Jialiang Tang helped resolve charging issues in sodium-ion batteries
that have prevented the technology from advancing to industry testing and use.
Yesterday scientists from Purdue University, Indiana, revealed some breakthrough research that shows how manufacturers can use Sodium in the place of Lithium and that fixes the potentially explosive issue and holds a charge properly.
As the Purdue newsroom report on the discovery states "The problem is that Sodium ions stick to the hard carbon end of a battery, called an anode, during the initial charging cycles and not travel over to the cathode end. The ions build up into a structure called a 'solid electrolyte interface' (SEI)." Unfortunately the interface grows too large with the SEI hobbling the ability to charge the battery properly.
The fix to the above issues sounds remarkably simple. "Purdue researchers proposed using sodium as a powder, which provides the required amount of sodium for the solid electrolyte interface to protect carbon, but doesn’t build up in a way that it consumes sodium ions," explained the newsroom post. To prevent combustion problems the sodium powder is created in an Argon filled environment. The Sodium is 'melted' using ultrasound before being cooled into a powder and suspended in a hexane solution.
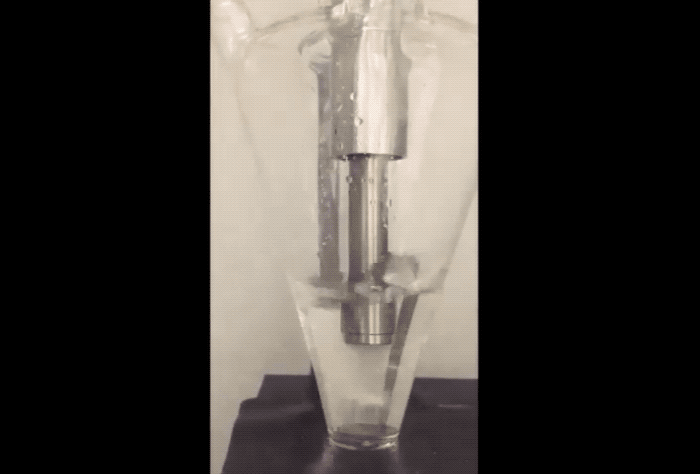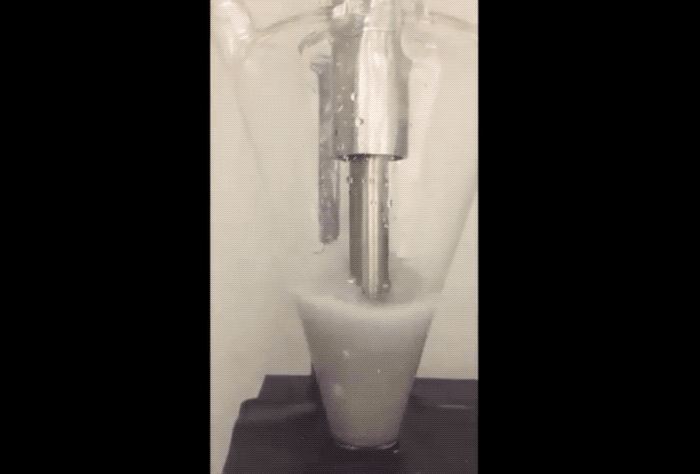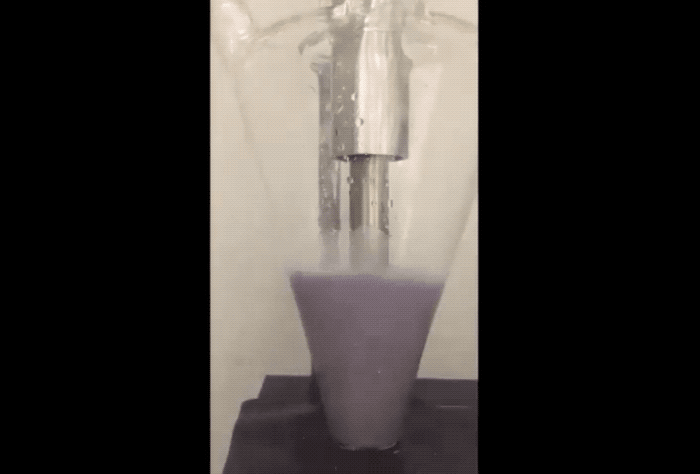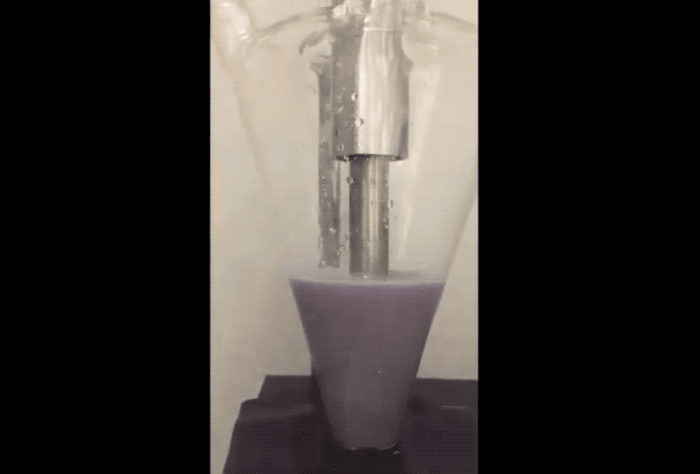
According to the team, adding just a few drops of the Sodium suspension onto the anode or cathode electrodes during their fabrication allows a Sodium-ion battery cell to charge and discharge with more stability and at higher capacity to provide a properly working battery.
Adding the fabricated Sodium powder during electrode processing "requires only slight modifications to the battery production process," asserted Vilas Pol, Purdue associate professor of chemical engineering. Thus this is a promising method to advance practical Sodium-ion battery technology.













